
The Balmer formula when written in terms of frequency of light is.
#ATOMIC SPECTRA LIGHT ENERGY AND ELECTRON STRUCTURE SERIES#
The Lyman series is in the ultraviolet region while Paschen and Brackett's series are found in the infrared region.There are other series of spectra for hydrogen which was discovered after their discoverers such as Lyman, Paschen, Brackett, and Pfund series, and these are represented by the given formulae:.Beyond this limit, no further distinct lines appear, and instead, only a faint continuous spectrum is seen. When n = ∞ is considered, one obtains the limit of series at λ = 364.6 nm and this is the shortest wavelength in the entire Balmer series.

This is also known as the Balmer formula.
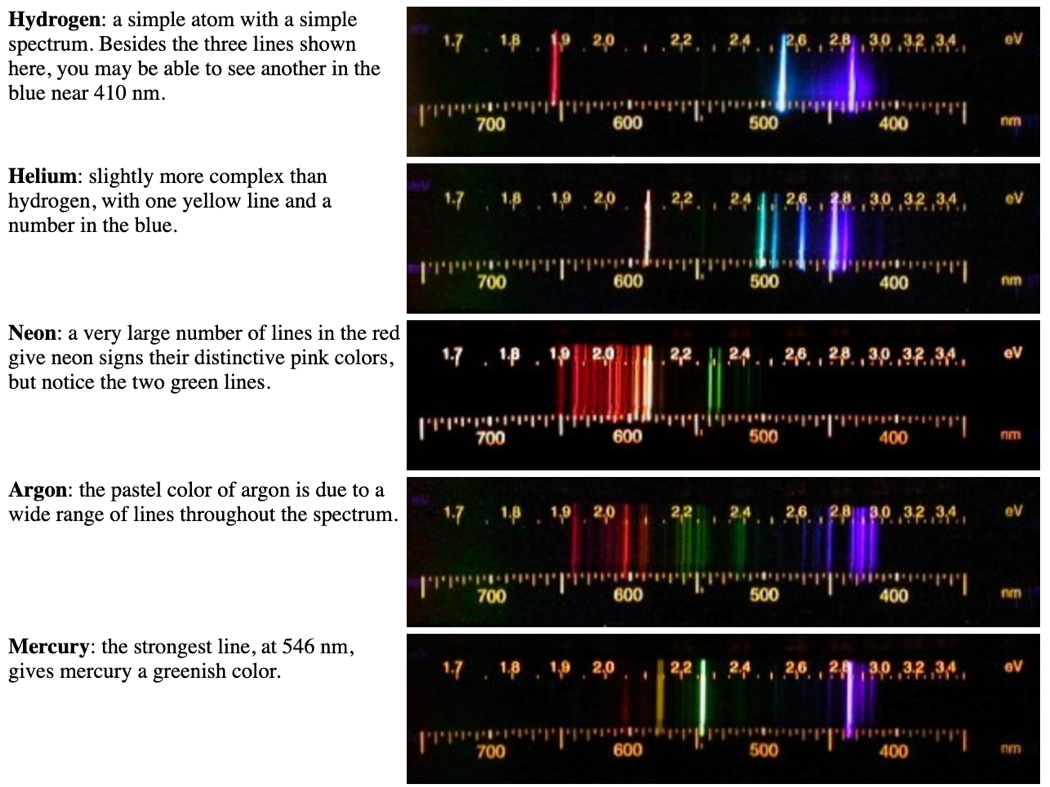
At first sight, there might not be any resemblance of order or regularity in spectral lines but the spacing between the lines within certain sets of hydrogen spectrum decreases regularly and each of these sets is known as spectral series. For instance, hydrogen is the simplest atom and thus, it has the simplest spectrum. The frequencies of the light emitted by a particular element exhibit a regular pattern.
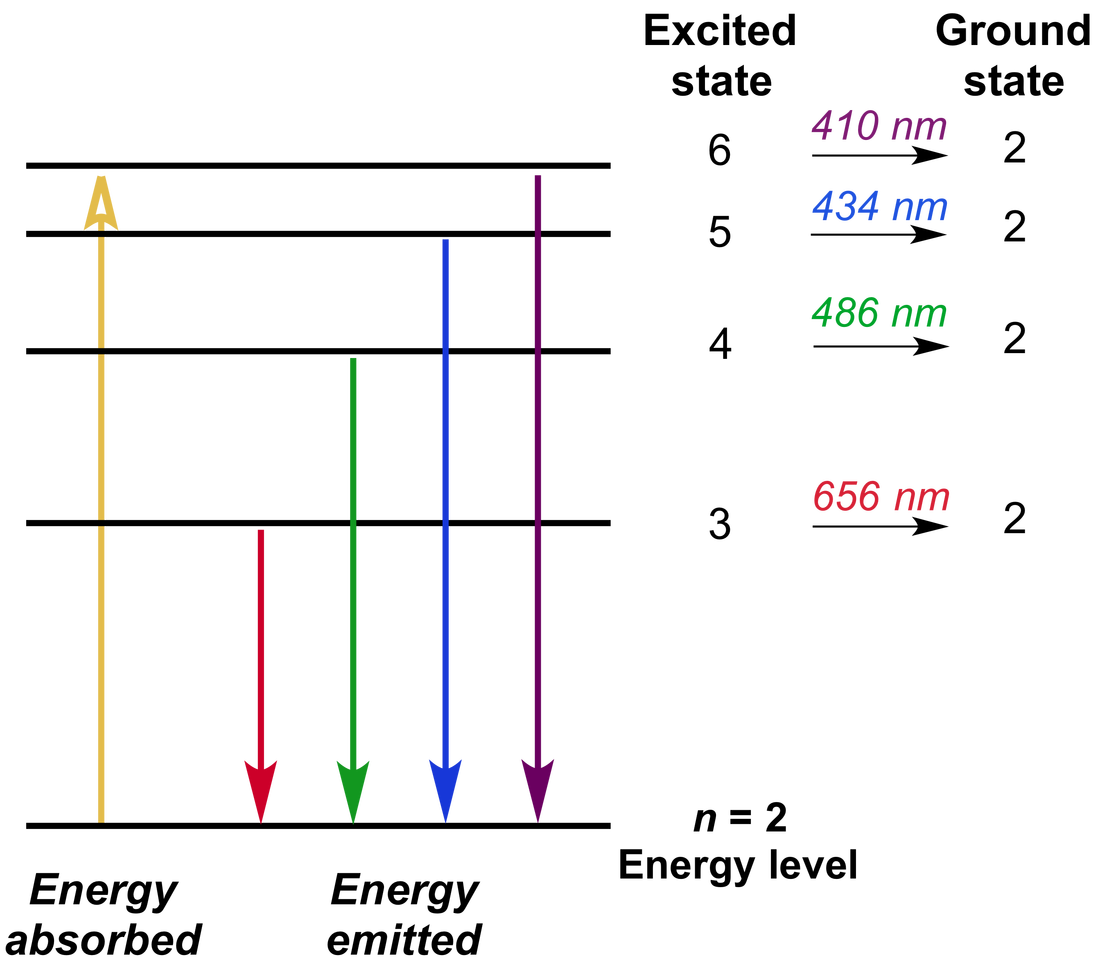
This is known as the absorption spectrum of the material of the gas.


 0 kommentar(er)
0 kommentar(er)
